Abstract
Background: Clinical data support the combination of an immunomodulatory drug, a proteasome inhibitor, and a glucocorticoid for the treatment of newly diagnosed multiple myeloma (NDMM). The combination of lenalidomide, bortezomib, and low-dose dexamethasone is a very active, well-tolerated standard of care in the transplant-ineligible NDMM population. Ixazomib, an oral proteasome inhibitor is approved by the US Food and Drug Administration in combination with lenalidomide and dexamethasone for the treatment of relapsed MM. Given that ixazomib has improved binding kinetics and pharmacologic profile compared with bortezomib, it is expected that these differences will translate into similar, if not improved, efficacy and safety profiles in the upfront setting. A phase l/ll study of lenalidomide, ixazomib, and dexamethasone in previously untreated patients with MM demonstrated that this combination was well-tolerated and active (NCT01217957). More recently, the GRIFFIN study evaluated the addition of the anti-CD38 monoclonal antibody, daratumumab to lenalidomide, bortezomib, and dexamethasone in transplant-eligible NDMM and demonstrated the ability of daratumumab to improve efficacy of the three-drug combination with an acceptable safety profile. Building upon these data, our study evaluates the addition of daratumumab to the all-oral regimen of lenalidomide, ixazomib, and dexamethasone in transplant-ineligible NDMM. The combination proposed in this study has been evaluated in NDMM irrespective of transplant eligibility and has demonstrated excellent efficacy and tolerability. Responses were rapid with 88% achieving a partial response (PR) or better after 2 cycles (33% very good partial response (VGPR) and 52% VGPR for 29 patients who had completed 4 cycles) with an overall response rate of 95%. 1 In our study, transplant-ineligible NDMM patients will be randomized to receive 12 cycles of induction with lenalidomide, ixazomib, dexamethasone, and daratumumab followed by lenalidomide alone versus lenalidomide, ixazomib, and daratumumab.
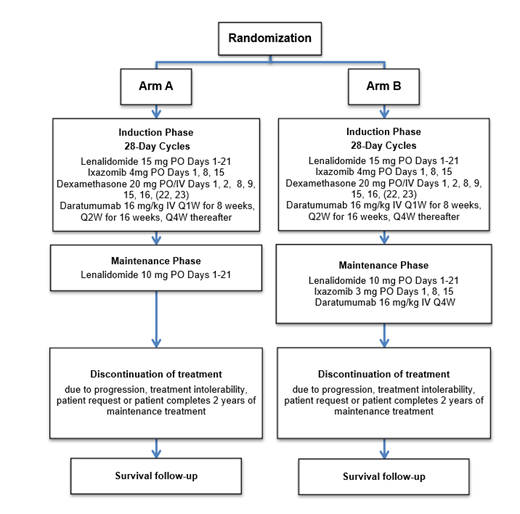
Study Design and Methods: A randomized, phase II clinical trial (NCT04009109) is being conducted to assess the impact on progression-free survival (PFS) of the addition of ixazomib and daratumumab to lenalidomide as maintenance treatment following induction with lenalidomide, ixazomib, dexamethasone, and daratumumab. In this ongoing trial, eligible patients are randomized 1:1 to Arm A (12 cycles of lenalidomide, ixazomib, dexamethasone, and daratumumab followed by lenalidomide until disease progression or unacceptable toxicity or a maximum of 2 years of maintenance therapy) vs. Arm B (12 cycles of lenalidomide, ixazomib, dexamethasone, and daratumumab, followed by lenalidomide, ixazomib, and daratumumab until disease progression or unacceptable toxicity or a maximum of 2 years maintenance therapy). Eligible patients have NDMM requiring treatment, are ≥18 years, have ECOG PS of 0-2, and must have been deemed ineligible for stem cell transplantation.
The primary endpoint is PFS. With a sample size of 188 patients (94 patients per arm) enrolled over a 36-month period and followed for a minimum of 24 months after the close of enrollment, a one-sided alpha=0.10 log rank test will have an 85% chance of detecting an increase in median PFS time from 30 months to 48 months (a hazard ratio of 0.625) with the addition of ixazomib and daratumumab to lenalidomide as a maintenance treatment following induction with ixazomib, lenalidomide, dexamethasone, and daratumumab. Secondary outcomes of interest include minimal residual disease, overall survival, changes in body composition, quality of life, psychosocial measures of functional status including social activity and support, psychological state, and nutrition using a geriatric assessment tool, and the association of these psychosocial measures with therapy-associated toxicities.
1Kapoor et al. Blood (2019) 134 (Supplement_1): 864.
Support: Alliance Foundation Trials; Janssen, Takeda, Celgene; https://acknowledgments.alliancefound.org
O'Donnell: Bristol Myer Squibb: Consultancy; Janssen: Consultancy; Takeda: Consultancy; Oncopeptide: Consultancy; Adaptive: Consultancy; Karyopharm: Consultancy. Nadeem: GSK: Consultancy; Karyopharm: Consultancy; Adaptive: Consultancy; Takeda: Consultancy; Bristol Myer Squibb: Consultancy. Dinner: Kite/Gilead: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Yee: Takeda: Consultancy; Sanofi: Consultancy; Bristol Myers Squibb: Consultancy; Oncopeptides: Consultancy; Janssen: Consultancy; Karyopharm: Consultancy; Amgen: Consultancy; Adaptive: Consultancy; GSK: Consultancy. Mo: Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Eli Lilly: Consultancy. Tuchman: Karyopharm: Research Funding; Shattuck Labs: Consultancy; Sanofi / Genzyme: Consultancy, Research Funding; Caelum: Consultancy, Research Funding; Oncopeptides: Consultancy. Raje: Caribou: Other; Janssen: Other; bluebird bio: Other; Amgen: Other; Celgene: Other; BMS: Other. Richardson: AstraZeneca: Consultancy; Regeneron: Consultancy; Janssen: Consultancy; Celgene/BMS: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Oncopeptides: Consultancy, Research Funding; GlaxoSmithKline: Consultancy; Secura Bio: Consultancy; Sanofi: Consultancy; Protocol Intelligence: Consultancy; Karyopharm: Consultancy, Research Funding; AbbVie: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal